Kova Resources
Laboratory Professionals

Proper collection, transportation, and handling of urine specimens are important to avoid contamination, or deterioration of constituents. This is where the complete KOVA® Urinalysis System differentiates itself from conventional urinalysis products. It is designed to eliminate significant sources of variation, assuring the right precision and accuracy, test after test. It includes everything the lab needs to standardize critical steps from sample collection to centrifugation, decanting and resuspension of sediment, slide preparation and reporting results.
What Makes the KOVA® System Components Unique?
- Secure Fit
The secure fit of the KOVA® Tubes and Caps prevent spillage during specimen collection, transportation, and handling, minimizing biohazardous exposure as well as reducing the risk of aerosol contamination during centrifugation.
- Unique Petters
The unique KOVA® Petters assure volume consistent sediment collection and suspension with one-step, contamination-free decanting.
- Precision Manufacturing
The precision manufactured KOVA® Glasstic 10 Slides feature specially designed wells for precise volume control, as well counting grids, eliminating variables that commonly interfere with microscopic analysis. Other slide products can only hold one or two samples, have seams between wells that increase the risk of sample cross-contamination, tend to break more easily, or disperse smaller particles to the edges due to adhesion forces, resulting in miscounts.
- Lab Standards
The KOVA® system conforms to CLIA (Clinical Laboratory Improvement Amendments) and (CLSI) Clinical and Laboratory Standards Institute Guidelines.
How to Use the KOVA® System
The KOVA® System is so easy that an untrained laboratory technician with less than 30 minutes of instruction can obtain the same results as an experienced technologist. It includes specially engineered microscopic slides, sample tubes & caps, custom pipettes or “petters”, collection cups and multi-level urinalysis controls for reliable, simple and efficient urinalysis testing and standardization.

Centrifuging
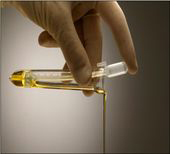
Decanting
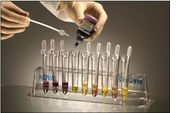
Resuspension
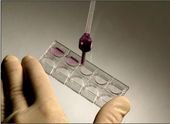
Slide Preparation

Sediment Suspension
Count
For Clinicians

The KOVA® Urinalysis System includes everything the lab needs to standardize critical steps from sample collection to centrifugation, decanting and resuspension of sediment, slide preparation, and quality control. Why is this relevant to you?
Between 32% and 75% of all testing errors in urinalysis occur in the preanalytical phase*. Proper collection, transportation, and handling of urine specimens are important to avoid contamination, or deterioration of constituents. The secure fit of the KOVA® Tubes and Caps prevent spillage during specimen collection, transportation, and handling, minimizing biohazardous exposure as well as reducing the risk of aerosol contamination during centrifugation.
The unique KOVA® Petters assure volume consistent sediment collection and suspension with one-step, contamination-free decanting.
The precision manufactured KOVA® Glasstic Slides feature specially designed wells for precise volume control, as well counting grids, eliminating variables that commonly interfere with microscopic analysis. slide-grid-detail-pop-out KOVA® Stain is a modified Sternheimer-Malbin stain that provides higher contrast for rapid and accurate identification of urinary sediment particles.
To assure the proper daily QC of macroscopic, microscopic, and chemical analysis of your patient’s urine specimens KOVA® offers KOVA-Trol® and KOVA Liqua-Trol®, urine-based controls that simulate both normal and abnormal levels of key analytes, as well as microscopics for sediment analysis.
The KOVA® Urinalysis System conforms to CLIA (Clinical Laboratory Improvement Amendments) and (CLSI) Clinical and Laboratory Standards Institute Guidelines.
This is where the complete KOVA® Urinalysis System differentiates itself from conventional urinalysis products. Ask if your laboratory uses the KOVA® Urinalysis System to assure better results.
For Patients

Analysis of urine specimens is the most commonly performed laboratory test in medicine today. Urine can be evaluated for:
Urinalysis is used to screen for kidney infections, stones, or disease, diabetes mellitus, metabolic diseases, liver problems, hypertension, liver disease, urinary tract infection, lupus, congestive heart failure, cancer, muscle breakdown, blood in the urine, and various other related conditions.
KOVA® International developed the KOVA® Urinalysis System, which consists of specially designed tubes, caps, decanters, slides, stains and QC products to standardize the critical steps from sample collection to analysis in the lab. The KOVA® Urinalysis System reduces significant sources of error, assuring that the doctor gets more dependable results to support your diagnosis and treatment. Ask if your laboratory uses the KOVA® Urinalysis System to assure better results.
For more information about urinalysis:
Resources
Publications, journal articles and materials regarding urinalysis.
